Abstract
Background: The current standard first-line systemic therapy for symptomatic/high-burden FL and MZL includes immunochemotherapy, most commonly rituximab with bendamustine. It has significant shortcomings: it relies on an alkylating agent with associated toxicities, is not curative, and leads to prolonged T-cell depletion, with potential long-term impairment of cellular immunity [Martinez-Calle N et al., BJH 2019]. Replacing bendamustine with the immunomodulatory drug lenalidomide at full doses did not lead to improved efficacy or toxicity in the RELEVANCE trial [Morschhauser et al., NEJM 2018]. However, in the Swiss/Nordic Lymphoma Group study [Zucca et al, Blood 2019] a significant improvement in complete response (CR, including CR unconfirmed) and progression-free survival (PFS) over rituximab alone was achieved with addition of much lower doses of lenalidomide (15mg). Low-dose lenalidomide may enhance cellular immunity via stimulation of effector (cytotoxic) T-cells and NK cells while inhibiting the immunosuppressive Treg population [Chang et al., Blood 2006].
Mosunetuzumab, a CD20xCD3-binding bispecific antibody has shown high single-agent activity in relapsed/refractory (R/R) FL and MZL, and was approved by the European Medicines Agency for R/R FL. In a phase 2 trial, mosunetuzumab resulted in a CR rate of 60% and overall response rate (ORR) of 80% in patients with R/R FL [Budde et al., Lancet Oncol, 2022], all of whom had prior exposure to alkylating agents, and many had received PI3K inhibitors, stem cell transplantation, chimeric antigen receptor T-cell therapy, and other immunosuppressive therapies.
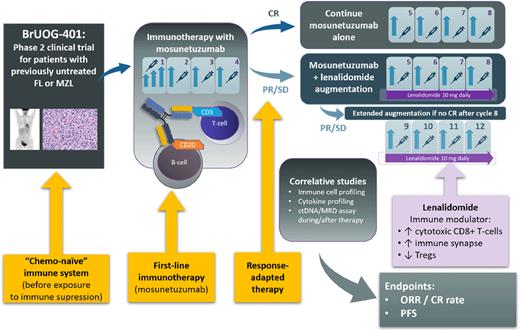
The Brown University Oncology Research Group BrUOG-401/ML43251 trial (NCT04792502) is an investigator-initiated phase 2 trial exploring a novel therapeutic approach for first-line treatment of FL/MZL. The study is based on several innovative hypotheses: 1) that treatment with immune effector cell-activating agent (mosunetuzumab) may lead to a high and durable rate of CR, and possibly eradication, of FL/MZL when applied before patients are exposed to traditional immunosuppressive modalities; 2) that low-dose lenalidomide may augment the cytotoxic T-cell-based response for patients who do not achieve CR with mosunetuzumab alone, and 3) that baseline parameters of host immune potential, measured in treatment-naïve state, can be predictive of success of bispecific antibody therapy.
Study Design and Methods: This multi-center, single-arm, phase 2 clinical trial (see Figure) is enrolling patients with FL and MZL in need of first-line systemic therapy. Patients with FL must meet the Groupe d'Etude des Lymphomes Folliculaires (GELF) criteria of high-burden disease as applied in the RELEVANCE trial [Morschhauser et al., NEJM 2018], whereas patients with MZL must require systemic therapy in the investigators' opinion. Treatment is structured as a response-adapted regimen. All patients will start with 4 cycles of subcutaneous mosunetuzumab monotherapy, using step-up dosing during Cycle 1 (5mg → 45mg → 45mg) to mitigate the risk of cytokine release syndrome (CRS). Patients attaining CR (according to Lugano criteria) after Cycle 4 will continue for a total of 8 cycles of mosunetuzumab monotherapy. Patients attaining partial response (PR) or stable disease (SD) will receive 4 additional cycles of mosunetuzumab in combination with low-dose lenalidomide (10mg daily continuously), with further restaging after Cycle 8, and additional 4 cycles of mosunetuzumab/lenalidomide if CR after C8 is not attained. The primary endpoints include the ORR and CR rate at final response assessment, and rates of toxicities, particularly CRS, tumor flare, and other immune-mediated adverse events. PFS and rate of progression of disease at 24 months (POD24) are among secondary endpoints. The study uses Simon's two-stage design, with a total planned sample size of 52 patients and the null hypothesis that the CR rate is 53% (as observed with immunochemotherapy in the RELEVANCE trial). Correlative studies aim at determining predictive biomarkers of response to first-line mosunetuzumab therapy (± lenalidomide augmentation), which include immune signatures based on circulating T-cell and macrophage subsets and serum cytokines. The investigators will further analyze changes in the circulating tumor DNA (ctDNA) during first-line immunotherapy with mosunetuzumab in this setting.
Disclosures
Olszewski:Precision Bio: Research Funding; Adaptive Biotechnologies: Research Funding; Celldex: Research Funding; Acrotech Biopharma: Research Funding; Schrodinger: Consultancy; TG Therapeutics: Consultancy, Research Funding; Genmab: Consultancy, Research Funding; Genentech: Research Funding. Huntington:Epizyme: Consultancy, Honoraria; ADC Therapeutics: Consultancy, Honoraria; AstraZeneca: Consultancy; Beigene: Consultancy; FlatonIron Health: Consultancy; AbbVie: Consultancy; Genentech: Consultancy; Merck: Consultancy; Seattle Genetics: Consultancy, Honoraria; Tyme: Consultancy; Pharmacyclics: Honoraria; AstraZeneca: Consultancy; Arvinas, Novartis, Servier, Bayer, SeaGen: Consultancy; Debiopharm Group: Research Funding; Agios: Research Funding; Janssen: Consultancy; TG Therapeutics: Consultancy, Research Funding; DTRM Biopharm: Research Funding; Celgene: Research Funding.
OffLabel Disclosure:
Mosunetuzumab - experimental agent. Lenalidomide is currently approved for second-line therapy of follicular lymphoma.
Author notes
Asterisk with author names denotes non-ASH members.